Vaccine injury related to Covid 19 jab is ignored by the government and pharmaceutical manufacturers. Numerous reports in the scientific literature indicate a growing concern over side effects and injury related to the Covid 19 vaccines or “jab.” However, CDC and FDA are slow-rolling the public report of these injuries and side effects, censoring the information to promote the narrative that everyone, including babies, should get the “jab.”
On the NIH’s Covid 19 website, an official website of the United States Government, there is a timeline for developing the Covid 19 Vaccines. It is self-aggrandizing in that they describe, “Decades of NIH-supported laboratory research laid the groundwork for the rapid development of mRNA vaccines in the first 100 days of the Covid-19 pandemic.”
From the NIH website: Covid timeline
- December 31, 2019 — The first cluster of people sick with what is now called COVID-19 is reported in Wuhan, China. The global response begins almost right away. The U.S. government has come together with private, non-governmental, and academic organizations to begin work on COVID-19 vaccines. Source
- January 2020 — Chinese scientists share the first genetic sequence of SARS-CoV-2 with the NIH database GenBank. Scientists from NIH and Moderna quickly pivot from studies of other viral vaccines to focus on a vaccine candidate for COVID-19, mRNA-1273, to respond to the outbreak. Source 1 Source 2
- March 11, 2020 — The World Health Organization (WHO) declares COVID-19 a pandemic. Source
- March 16, 2020 — NIH clinical trials for the Moderna mRNA vaccine begin. Source
- April 17, 2020 — NIH launches Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV), a first-of-its-kind public-private partnership for developing COVID-19 treatments and vaccines. Source
- May 15, 2020 — Operation Warp Speed launches to coordinate federal government efforts that speed up the approval and production of reliable COVID-19 diagnostics, vaccines, and treatments. Source 1 Source 2
- July 8, 2020 — NIH launches the COVID-19 Prevention Network (CoVPN), which uses the existing structure of NIH clinical trial networks to support trials of COVID-19 vaccines and other prevention tools. Source
- November 16, 2020 — A large-scale Phase 3 clinical trial of the Moderna mRNA vaccine shows promising interim results. Source
- December 11, 2020 — The FDA grants an emergency use authorization (EUA) to the Pfizer-BioNTech mRNA vaccine for people age 16 and older. Source
- December 18, 2020 — The FDA grants an EUA to the Moderna mRNA vaccine for people age 18 and older. Source
- August 23, 2021 — The FDA grants full approval to the Pfizer-BioNTech mRNA vaccine for people age 16 and older. Source
- October 29, 2021 — The FDA grants an EUA to the Pfizer-BioNTech mRNA vaccine for children age 5 to 11. Source
- January 31, 2022 — The FDA grants full approval to the Moderna mRNA vaccine for people age 18 and older. Source
- March 14, 2022 — NIH launches Phase 1 clinical trials for three mRNA HIV vaccines. These vaccines apply lessons learned from the development of mRNA vaccines for COVID-19. Source
- March 2022 — Data show that the U.S. COVID-19 vaccination program is estimated to have prevented 2 million deaths, 17 million hospitalizations, and 66 million infections through March 2022. Vaccination is also estimated to have saved nearly $900 billion in health care costs. Source
- June 17, 2022 — The FDA grants an EUA to the Pfizer-BioNTech and Moderna mRNA vaccines for children age 6 months or older. Source
- July 11, 2022 — NIH launches a Phase 1 clinical trial for an mRNA Nipah virus vaccine. Source
- August 31, 2022 — The FDA grants an EUA of the Moderna and Pfizer-BioNTech COVID-19 vaccines to authorize bivalent formulations for use as a booster dose. These updated boosters contain mRNA components for both the original strain of SARS-CoV-2 and its Omicron variant. Source
- December 8, 2022 — The FDA grants an EUA to the Pfizer-BioNTech and Moderna bivalent COVID-19 vaccines for children age 6 months or older. Source
Selected Adverse Events Reported after Covid-19 Vaccination, from CDC.gov
CDC is providing timely updates on the following adverse events of interest:
- Anaphylaxis after COVID-19 vaccination is rare and has occurred at a rate of approximately 5 cases per one million vaccine doses administered. Anaphylaxis, a severe type of allergic reaction, can occur after any kind of vaccination. If it happens, healthcare providers can effectively and immediately treat the reaction. Learn more about COVID-19 vaccines and allergic reactions, including anaphylaxis.
CDC scientists have conducted detailed reviews of cases of anaphylaxis and made the information available to healthcare providers and the public:
- Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine
- Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US—December 14, 2020-January 18, 2021
- Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine— United States, December 21, 2020-January 10, 2021
- Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine — United States, December 14-23, 2020
- Thrombosis with thrombocytopenia syndrome (TTS) after J&J/Janssen COVID-19 vaccination is rare and has occurred in approximately 4 cases per one million doses administered. TTS is a rare but serious adverse event that causes blood clots in large blood vessels and low platelets (blood cells that help form clots).
A review of reports indicates a causal relationship between the J&J/Janssen COVID-19 vaccine and TTS. CDC scientists have conducted detailed reviews of TTS cases and made the information available to healthcare providers and the public:
- US Case Reports of Cerebral Venous Sinus Thrombosis With Thrombocytopenia After Ad26.COV2.S Vaccination, March 2 to April 21, 2021
- Case Series of Thrombosis with Thrombocytopenia Syndrome following COVID-19 vaccination—United States, December 2020–August 2021
- Updates on Thrombosis with Thrombocytopenia Syndrome (TTS) [1.3 MB, 39 Pages]
- Guillain-Barré Syndrome (GBS) in people who have received the J&J/Janssen COVID-19 vaccine is rare. GBS is a rare disorder where the body’s immune system damages nerve cells, causing muscle weakness and sometimes paralysis. GBS has largely been reported in men ages 50 years and older.
Based on a recent analysis of data from the Vaccine Safety Datalink, the rate of GBS within the first 21 days following J&J/Janssen COVID-19 vaccination was found to be 21 times higher than after Pfizer-BioNTech or Moderna (mRNA COVID-19 vaccines). After the first 42 days, the rate of GBS was 11 times higher following J&J/Janssen COVID-19 vaccination. The analysis found no increased risk of GBS after Pfizer-BioNTech or Moderna (mRNA COVID-19 vaccines). Similarly, CDC found higher than expected rates of GBS reported to the Vaccine Adverse Event Reporting System (VAERS) after J&J/Janssen COVID-19 vaccination but not after mRNA COVID-19 vaccination. CDC and FDA will continue to monitor for and evaluate reports of GBS occurring after COVID-19 vaccination and will share more information as it becomes available.
- Myocarditis and pericarditis after COVID-19 vaccination are rare. Myocarditis is inflammation of the heart muscle, and pericarditis is inflammation of the outer lining of the heart. Most patients with myocarditis or pericarditis after COVID-19 vaccination responded well to medicine and rest and felt better quickly. Most cases have been reported after receiving Pfizer-BioNTech or Moderna (mRNA COVID-19 vaccines), particularly in male adolescents and young adults.
A review of vaccine safety data in VAERS from December 2020–August 2021 found a small but increased risk of myocarditis after mRNA COVID-19 vaccines. Over 350 million mRNA vaccines were given during the study period and CDC scientists found that rates of myocarditis were highest following the second dose of an mRNA vaccine among males in the following age groups:
- 12–15 years (70.7 cases per one million doses of Pfizer-BioNTech)
- 16–17 years (105.9 cases per one million doses of Pfizer-BioNTech)
- 18–24 years (52.4 cases and 56.3 cases per million doses of Pfizer-BioNTech and Moderna, respectively)
- Multiple studies and reviews of data from vaccine safety monitoring systems continue to show that vaccines are safe. As a result, the agency will refocus enhanced surveillance and safety monitoring efforts toward children and adolescents.
As of March 2, 2023, there have been 1,059 preliminary reports in VAERS among people younger than age 18 years under review for potential cases of myocarditis and pericarditis. Of these, 244 remain under review. Through confirmation of symptoms and diagnostics by provider interview or review of medical records, 715 reports have been verified to meet CDC’s working case definition for myocarditis. See below for counts of verified reports of myocarditis by age group.
- 5-11 years: 23 verified reports of myocarditis after 23,376,785 doses administered
- 12-15 years: 376 verified reports of myocarditis after 25,913,772 doses administered
- 16-17 years: 316 verified reports of myocarditis after 14,180,263 doses administered
As the COVID-19 vaccines are authorized for younger children, CDC and FDA will continue to monitor for and evaluate reports of myocarditis and pericarditis after COVID-19 vaccination and will share more information as it becomes available. Learn more about myocarditis and pericarditis, including clinical considerations, after mRNA COVID-19 vaccination.
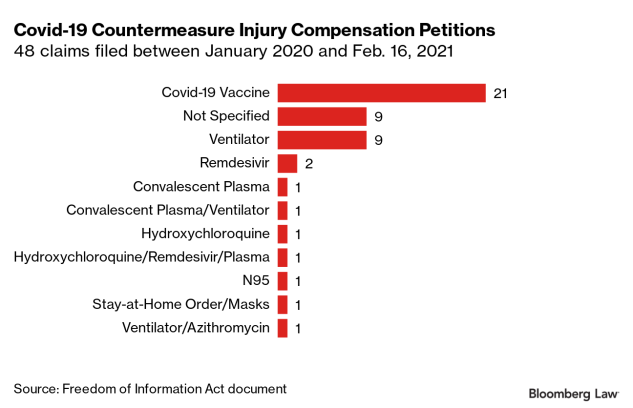
- Reports of death after COVID-19 vaccination are rare. FDA requires healthcare providers to report any death after COVID-19 vaccination to VAERS, even if it’s unclear whether the vaccine was the cause. Reports of adverse events to VAERS following vaccination, including deaths, do not necessarily mean that a vaccine caused a health problem. More than 672 million doses of COVID-19 vaccines were administered in the United States from December 14, 2020, through March 1, 2023. During this time, VAERS received 19,476 preliminary reports of death (0.0029%) among people who received a COVID-19 vaccine. CDC and FDA clinicians review reports of death to VAERS including death certificates, autopsy, and medical records. Continued monitoring has identified nine deaths causally associated with J&J/Janssen COVID-19 vaccination. CDC and FDA continue to review reports of death following COVID-19 vaccination and update information as it becomes available.
Long Covid is acknowledged by the CDC website, but is still gathering information. It is known to be associated with many different conditions, and the mechanism is being investigated.
A cautionary statement from Immunology 2022 Apr;165(4):386-401.
doi: 10.1111/imm.13443. Epub 2022 Jan 7.,mk,
Recently, new-onset autoimmune phenomena after COVID-19 vaccination have been reported increasingly (e.g., immune thrombotic thrombocytopenia, autoimmune liver diseases, Guillain-Barré syndrome, IgA nephropathy, rheumatoid arthritis, and systemic lupus erythematosus). Molecular mimicry, the production of particular autoantibodies, and the role of certain vaccine adjuvants seem to be substantial contributors to autoimmune phenomena. However, whether the association between COVID-19 vaccine and autoimmune manifestations is coincidental or causal remains to be elucidated.
The statements from CDC are designed to reassure but do not reflect what researchers are seeing. Researchers are finding that the immunogenicity of the mRNA vaccines is persistent for months to years, and the ultimate side effects are still unknown. Unfortunately, because of the emergency use authorization by FDA, the mRNA vaccine manufacturers were granted liability for adverse events related to the vaccines.